On page 50 of our Pool & Spa Operator Handbook in chapter 5, there is an excellent chart that lists all the chlorine compounds and their respective characteristics/properties. One of the properties listed is “Available Chlorine Content.” You should remember that all chlorine compounds — namely, liquid chlorine, Trichor, Dichlor and such — are NOT created equal!
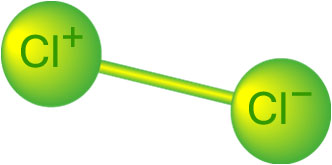
The Chlorine Gas molecule has one Cl+ atom (oxidizing power) and one Cl- atom (inert, no oxidizing power)
The term “Available Chlorine Content” (ACC) was established to compare the potential oxidizing power of the various chlorine compounds. Chlorine gas is defined as containing 100% available chlorine and is used as the basis for determining chlorine content in the other compounds. “The available chlorine content of a material is determined by the amount of iodine (I2) which the material liberates from an acidified iodide solution. The calculated weight of elemental chlorine (Cl2) required to liberate the same amount of iodine is the Available Chlorine Content of the material.” 1
Chlorine, in its elemental gaseous form, contains two (2) atoms — which means that is it “diatomic.” Only one of the atoms, however, (Cl+) has the oxidizing power; the other atom (Cl-) is inert (non-oxidizing). This means that only half of the chlorine gas molecule, by weight, has oxidizing potential.
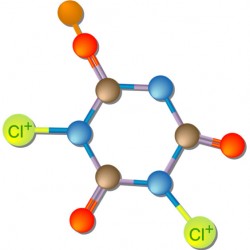
The Anhydrous DiChlor Molecule. It contains 2 Cl+ atoms, both have oxidizing power
The Dichlor compound (the anhydrous form – C3N3O3NaCl2) on the left is made up of the cyanuric acid molecule (C3N3O3), one sodium atom (Na) and two chlorine (Cl2) atoms. If you compare the weight of the Cl2 with the weight of all the other elements in anhydrous Dichlor, you will discover that only 32.23% of it is actually chlorine. However, the chart on page 50 of our Pool & Spa Operator Handbook, “Characteristics of Disinfectants,” lists anhydrous Dichlor with 63% available chlorine content. Why?
Well, it’s because both the chlorine atoms (Cl2) in Dichlor are Cl+ (i.e. have oxidizing power). Only one of the atoms of Cl2 in chlorine gas has oxidizing power. So, this means that the Cl2 in Dichlor has twice the “oxidizing power” (i.e. available chlorine content) as the Cl2 in chlorine gas.
 All the chorine elements in all the chlorine compounds are Cl+, so you need to double the weight percentage of chlorine in each compound to get the available chlorine content.
All the chorine elements in all the chlorine compounds are Cl+, so you need to double the weight percentage of chlorine in each compound to get the available chlorine content.
Here’s a helpful chart that lists various chlorine compounds, their equivalent weight to one pound of chlorine gas, their weight in a “one pound DE scoop” and a few other things. Download it, put it in your truck, and USE IT! You may be overdosing — you might save some money!
1 OxyChem’s FAQ about Chlorinated Isocyanurates
______________________________________
Our CPO class this past Thursday and Friday, March 8-9 at PoolCorp’s Superior Anaheim was fun and exciting. We had another mixture of pool guys and county pool inspectors, and it was a very lively class with information passed back and forth. Thank you all for being there! By working together, we WILL create a healthier and safer swimming environment for all!
Thanks again to all at PoolCorp, and a special thanks to Brian and Steve in Anaheim for carrying our equipment and supplies upstairs!

Comments
Very clear and simple explanation of a very complicated subject for most people — at least, those of us that are NOT chemistry majors!
Truly this is a very clear, concise explanation of the ACC concept, excellent work, thanks again for putting this together for us,
Paul
Apollo Pools in Canada